
Labeling of biocidal products
| Subject | - Individuals manufacturing/importing biocidal products which have received product approval for the purpose of domestic sales or distribution |
| Labeled Facts | - Composition/mixture ratio of all biocidal substances used in product - Name/ trade name, address and contact information of manufacturer/importer - Dangers accompanying use of product and first aid directions for emergencies - Date of expiry and disposal methods - If nanomaterials are included by design, name, purpose and uses of said nanomaterial(s) - Miscellaneous facts |
| Labeling Method | - In Korean in principle(if foreign language is also used, must be in smaller print) - Must be in color that contrasts with background - Must have no risk of becoming erased or detached |
※Example of label
| Name of Product | 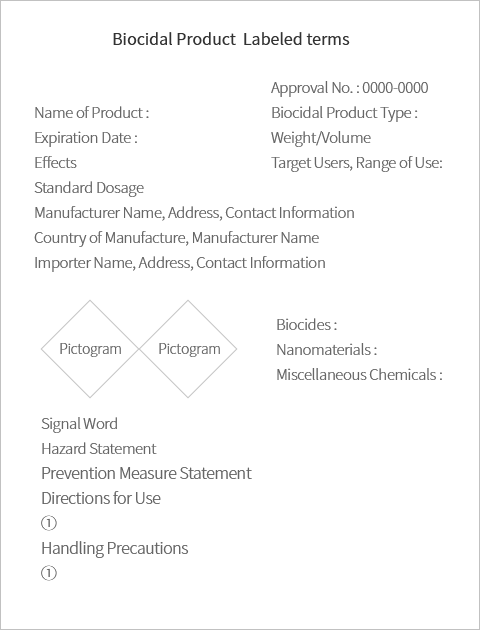 |
| Biocidal Product Type | |
| Expiration Date | |
| Weight/Volume | |
| Effects | |
| Target Users, Range of Use | |
| Standard Dosage | |
| Manufacturer Name, Address, Contact Information (only for domestically manufactured products) | |
| Country of Manufacture, Manufacturer Name (only for imported products) | |
| Importer Name, Address, Contact Information (only for imported products) | |
| Sign for Child-Resistant Packaging (only for products required to use child-resistant packaging) | |
| Biocides | |
| Nanomaterials | |
| Miscellaneous Chemicals | |
| Hazard/Danger of Product | |
| Directions for Use | |
| Handling Precautions | |
| Approval No. | |
| Manufacture No. | |
| Labeled terms | Example |
|---|
Safety and labeling standards for biocide treated products
| Subject | - Individuals manufacturing/importing biocide treated products for the purpose of domestic sales or distribution |
| Safety Standards | - Must use biocidal products which have received product approval - When importing biocide treated products, it must use biocidal products which satisfy similarity standards* with biocidal products which have received product approval |
| Terms to be labeled according to standards | - Notice that biocidal products were used - Names and functions of all biocidal substances contained in biocidal products used in biocide treated product - If nanomaterials are included by design in biocidal products used, notice that said nanomaterials are included - Dangers and handling precautions for biocidal products used in biocide treated product |
| Labeling Method | - In Korean in principle(if foreign language is also used, must be in smaller print) - Must be in color that contrasts with background - Must have no risk of becoming erased or detached |
*Based on similarity
· If the biocidal substance was used as an approved biocidal product type
· If the biocidal substance was notified by the government
· If the biocidal substance was publicly recognized by a foreign government as being safe when utilized for the given use
· If the safety of the biocidal product used in the treated product has been recognized by a foreign government’s approval, confirmation, etc.
Interim Measures
Manufacturers/importers of biocide treated products may manufacture/import them without following safety and labeling standards until their respective interim measure periods end.
| Group | Classification according to interim measures | Interim measure period |
|---|---|---|
| 1 | If all biocidal substances in all biocidal products used on the biocide treated product are existing biocides subject to grace period for approval (if the biocide treated product is imported, includes cases where all used biocidal products satisfy similarity standards) | Within 2 years from the last end date of all interim measure periods for biocidal products which only contain biocidal substances subject to grace period for approval |
| 2 | If all existing biocides in all biocidal products used in biocide treated product were prohibited from manufacture/import, or had their designation cancelled | Within 2 years from the manufacture/import prohibition date or the designation cancellation date, whichever comes first |
| 3 | Biocide treated products which do not fall under either Group 1 or 2 | Until December 31st, 2022 |
| 4 | Individuals manufacturing/importing biocide treated products in the periods as detailed in Groups 1~3, who have submitted data as demanded by presidential decree to explain reasons for why they cannot comply with the safety standards and/or labeling standards within the given time | May extend interim measure period by up to 1 year |
Example of interim measures
<Interim measures for manufacture/import of biocide treated products>
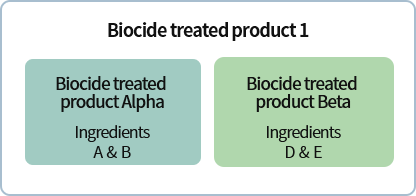
*Example for Group 1*Q.
Biocide treated product 1 uses biocidal products Alpha and Beta. If Alpha and Beta respectively contain existing biocides A/B and D/E which are subject to approval postponement, from what point onwards must the biocide treated product safety standards and labeling standards be observed?
A.
Out of the ingredients of biocidal products Alpha and Beta, the substance whose date of expiry for the approval postponement period comes last is substance E. Therefore, add said substance’s approval postponement period(8 years), the additional postponement period for product approval(2 years), and the postponement period for biocide treated products(2 years); the safety standards and labeling standards must be observed within 12 years.
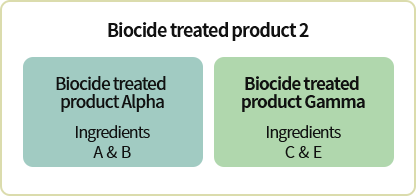
*Example for Group 2* Q.
Biocide treated product 2 uses biocidal products Alpha and Gamma. If Alpha and Gamma respectively contain existing biocides A/B and C/E which are subject to approval postponement, from what point onwards must the biocide treated product safety standards and labeling standards be observed?
A.
Among Alpha and Gamma’s ingredients, substance C’s designation as existing biocide will be cancelled starting 2021.7.30. Therefore, C is the substance whose date for manufacture/import prohibition or designation cancellation comes first, thus safety standards and labeling standards must be observed within 2 years from 2021.7.30.
| Ingredients | Notice Contents |
|---|---|
| A | Existing biocide subject to approval postponement (approval postponement period: 3 years) |
| B | Existing biocide subject to approval postponement (approval postponement period: 5 years) |
| C | Substance’s designation as existing biocide to be cancelled starting 2021.7.30. |
| D | Existing biocide subject to approval postponement (approval postponement period: 3 years) |
| E | Existing biocide subject to approval postponement (approval postponement period: 8 years) |