
The GHS(Globally Harmonized System of Classification and Labelling of Chemicals) is a system which aims to more efficiently convey information of chemical substances to workers and general users, by classifying the hazards/risks of chemical substances and using a standardized form of warning labels and MSDS in accordance with the international classification standards decided by the UNECE.
Major services provided in regards with GHS and MSDS![]() Investigation of information on MSDS/warning labels
Investigation of information on MSDS/warning labels![]() Preparation, review and translation of MSDS/warning labels under OSHA(Occupational Safety Health Act)
Preparation, review and translation of MSDS/warning labels under OSHA(Occupational Safety Health Act)![]() Provision of information on the regulatory status for paragraph 15 of the MSDS (Material Safety Data Sheet)
Provision of information on the regulatory status for paragraph 15 of the MSDS (Material Safety Data Sheet)
- OSHA
- Chemical Substances Control Act
- Act on the Safety Control of Hazardous Substances
- Wastes Control Act
- Clean Air Conservation Act
- Framework Act on Fire Service
- Water Environment Conservation Act
- Soil Environment Conservation Act
- Marine Environment Management Act
- High-Pressure Gas Safety Control Act
- Persistent Organic Pollutants Control Act
- Act on the Control of the Manufacture, Export and Import, etc. of Specific Chemicals and Chemical Agents for the Prohibition of Chemical and Biological Weapons
- Act on the Allocation and Trading of Greenhouse Gas Emission Permits
- Act on the Control of Manufacture of Specific Substances for the Protection of the Ozone Layer
- Youth Protection Act
- Regulation on Marine Transportation and Storage of Dangerous Products
- Regulation on Rail Transportation of Dangerous Products
- Technical Standard for Air Transportation of Dangerous Products![]() Submission of MSDS and confirmation dossiers under OSHA, application for approval of confidentiality
Submission of MSDS and confirmation dossiers under OSHA, application for approval of confidentiality
Preparation of MSDS and subjects excluded from submission
- Health functional foods defined by the “Health Functional Foods Act”
- Pesticides defined by the “Pesticide Control Act”
- Narcotic drugs and psychotropic drugs defined by the “Narcotics Control Act”
- Fertilizers defined by the “Fertilizer Control Act”
- Livestock feeds defined by the “Control of Livestock and Fish Feed Act”
- Raw materials defined by the “Act on Protective Action Guidelines Against Radiation in the Natural Environment”
- Products provided for use by the general public in daily life out of consumer chemical products and biocidal products defined by “Consumer Chemical Products and Biocides Safety Control Act”
- Foods and food additives defined by the “Food Sanitation Act”
- Drugs and quasi-drugs defined by the “Pharmaceutical Affairs Act”
- Radioactive substances defined by the “Nuclear Safety Act”
- Cleansing and hygiene products defined by the “Cleansing and Hygiene Products Control Act”
- Medical devices defined by the “Medical Devices Act”
- Advanced biopharmaceuticals defined by the “Act on the Safety of and Support for Advanced Regenerative Medicine and Advanced Biological Products”
- Explosives defined by the “Act on the Safety Management of Guns, Swords, Explosives, etc.”
- Waste defined by the “Wastes Control Act”
- Cosmetics defined by the “Cosmetics Act”
- Chemical substances or mixtures provided for use by the general public in daily life(including handling in the workplace)
- Chemical substances or products used in R&D as notified by the Minister of Employment and Labor
(only applicable for cases where annual manufacture/import quantity is under 100kg, individual containers must not exceed 10kg)
- Chemical substances recognized as having low risk of toxicity/explosiveness and thus notified by the Minister of Employment and Labor
Process for MSDS submission and substitute data entry examination
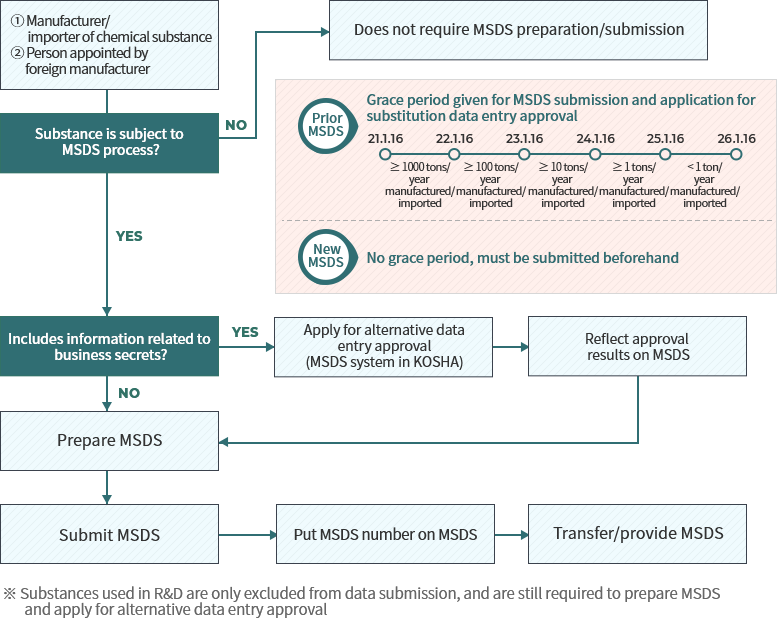
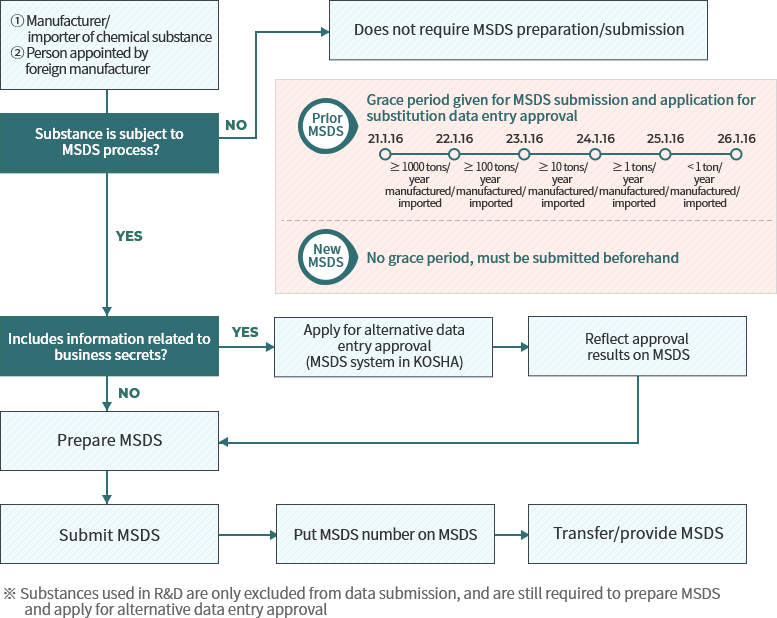
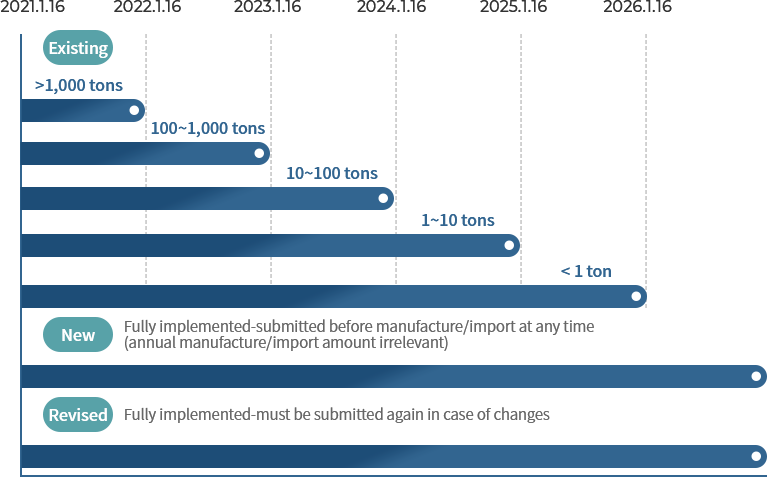
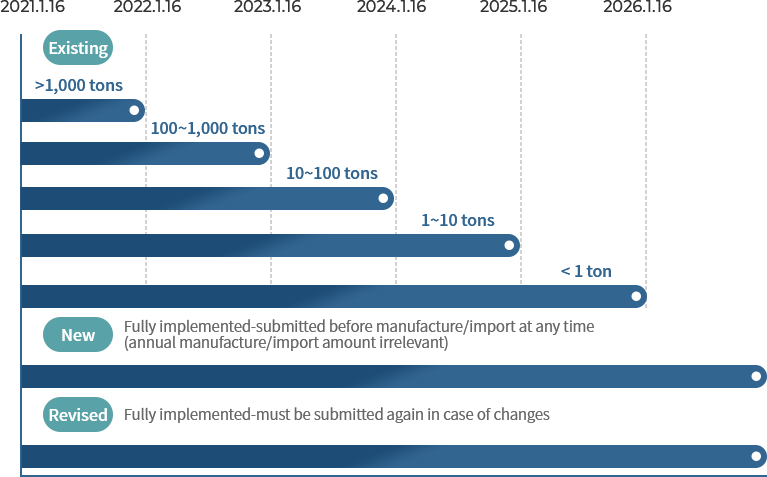
When to submit MSDS (based on annual manufacture/import tonnage of the substance)
MSDS substitute data entry examination
Previously, business owners were allowed to not enter their products’ constituents’ names and content on the MSDS if they judged on their discretion that there were reason to protect these facts as confidential data. However, in order to protect worker health and safety, under the amended law individuals who wish not to reveal constituent names and content must first seek approval from the Minister of Employment and Labor, and substitute names and content must be entered so that hazard/risk may be inferred upon exposure. Nonetheless, in case of chemicals used in R&D, these will be examined behind closed doors, have their required submission dossier simplified(omits validity for non-disclosure), and examination period will be shortened to under 2 weeks.
The valid term of approval is 5 years, and individuals wishing to extend this period must submit an application for extension approval and relevant documents through the MSDS system by 30 days before approval expiry.
Facts checked for prior approval
① Validity for non-disclosure
② Suitability of alternative data
③ Confirm appropriateness of MSDS
List of documents to be submitted
- Data that verifies information in question is confidential
1) Not known to the public
① Range of persons who know information in question
② Whether information in question has already been made public by another law
2) Secrecy protection
① Types and extent of measures taken by the manufacturer/importer of the information’s chemical, for the purpose of protecting its secrecy(restricting access to information, obligating employees to maintain secrecy and signing non-disclosure agreements with them, physical security measures, maintaining data processing environment with security systems, etc.)
② Ease of access and obtaining information in question by outsiders
3) Economic utility
① Benefits competing companies would gain if information in question were revealed to the public
② Effort and costs spent by information’s chemical’s manufacturer/importer to develop said information
- Substitute data
1) Evaluation standards for suitability of substitute name
2) Appropriate range of entry for substitute content
① If secret constituent’s content ≥25%, ±20%P(percent points)
② If secret constituent’s content <25%, ±10%P
- Name, content, health and environmental hazards, physical danger information for the chemical substance to be entered as substitute data
- MSDS
- Name and content of chemical substances not conforming to classification standards
- Miscellaneous information required for approval to use substitute data in place of substance’s name and content as decided and notified by the Minister of Employment and Labor
Substances excluded from entering substitute data on MSDS
- Substances prohibited from manufacture, etc. underOSHA
- Authorized substances under OSHA
- Hazardous substances subject to control under “Regulation on Industrial Safety Health Standards”
- Hazardous agents subject to working environment measurements under OSHA
- Hazardous agents subject to special health examinations under OSHA
- Chemical substance MSDS non-disclosure exceptions decided by K-REACH
Submission of information by appointee of foreign manufacturer
In case a foreign manufacturer is reluctant to provide information on the constituents of a product(s) to the importer, the manufacturer may designate an appointee to represent them in such tasks as MSDS preparation and submission, submission of chemical substance confirmation dossiers, approval for substitute data entry, valid term extension approval and formal objections, providing MSDS to importers, etc.