
Individuals wishing to manufacture or import biocides must receive approval for the substance before manufacture/import.
Approval of existing biocides
Individuals wishing to manufacture/import biocides which were contained in biocidal products or biocide treated products distributed domestically before December 31st, 2018 may continue to manufacture/import said biocide substance during the grace period if the substance was designated/notified as an existing biocide substance under grace period for approval.
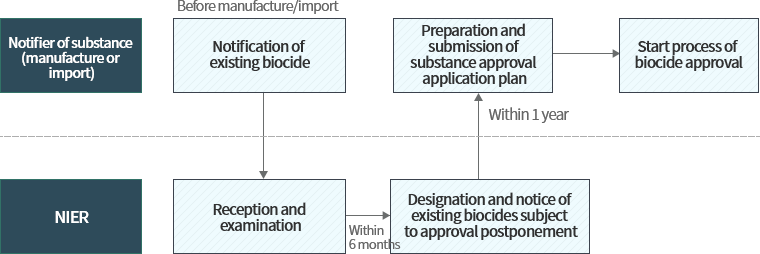
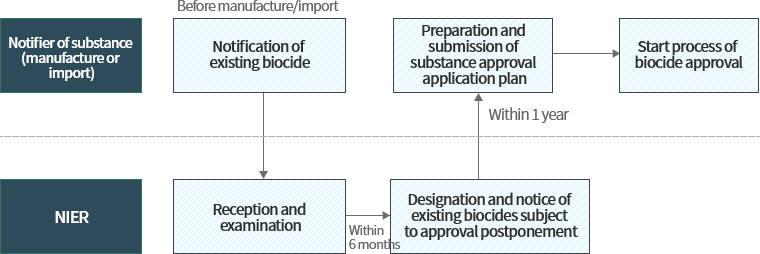
The biocide approval system divides biocides into approval-postponed substances and non-postponed substances. Biocides contained in products that were domestically distributed before 2018.12.31. are classified as existing substances, and “approval-postponed substances” are those substances designated/notified by the Ministry of Environment after considering their hazard/risk among existing substances notified to the Ministry. If the biocide is considered approval-postponed, a grace period is given depending on the type of the product where the substance is used, and the data required for approval must be jointly submitted through a CICO.
Non-postponed substances’ obligations for approval are only postponed until the designation/notification date for approval-postponed substances(December 31st, 2019). Afterwards these substances are prohibited from manufacture/import without biocide approval. New substances must receive approval before manufacture/import.
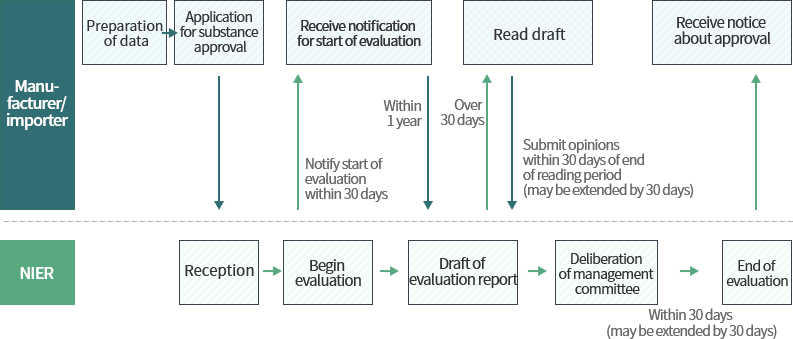
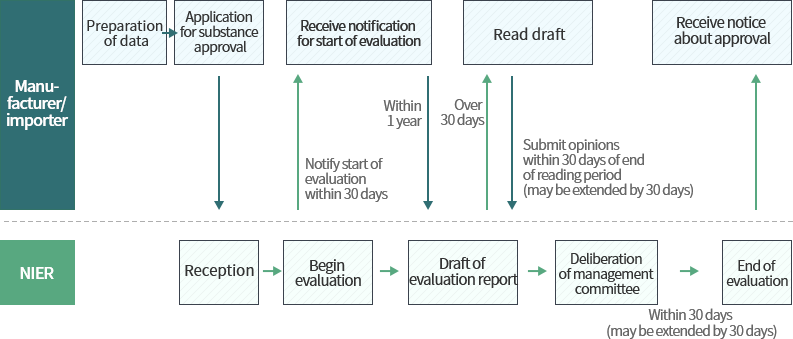
Approval procedure for biocides
Grace periods for approval of biocidal substances subject to grace period
| Grace Period | ’22.12.31 | ’24.12.31 | ’27.12.31 | ’29.12.31 |
|---|---|---|---|---|
| Type | Disinfectants | Control substances for other vertebrates | Product preservatives | Construction material preservatives |
| Algicides | Control substances for other invertebrates | Surface treatment preservatives | Material/equipment preservatives | |
| Rodenticides | Wood preservatives | Preservatives for fibers, leather, etc. | Embalming/taxidermy preservatives | |
| Insecticides | Antifoulants for ships/underwater equipment | |||
| Repellents |
Procedure for approval of existing biocides subject to grace period
Procedure for approval of substances and new substances not subject to grace period
Data required in biocide approval application
| Serial No. | Submitted Information | Details |
|---|---|---|
| 1 | Manufacturer/Importer information | - Name, trade name, address and contact information of applicant |
| 2 | Information of biocide | - Name, identifiable information(molecular formula, chemical composition, etc.) |
| 3 | Type of biocidal product | - Types of biocidal products in which the biocide in question may be used |
| 4 | Information on biocidal substance | - Physical/chemical/biological properties - Exposure information(uses, main exposure channels, form of exposure, etc.) - Hazard/risk information regarding health/animals/environment - Effects/efficacy - Classification and labeling |
| 5 | Data proving relaxed approval | - In case one or more relaxed approval conditions are applicable to substance, data which verifies said fact |
| 6 | Raw materials and processes used in manufacture of biocide | - Raw materials and processes used in manufacture of biocide - Handling precautions, disposal methods for biocide - Information for domestic/overseas use and restrictions imposed on biocide - Confirmation form for individual submission of data - In case of person(s) commissioning the manufacture of biocide, documents which verify said act such as copy of commission contract, etc. |
| 7 | Comprehensive data on safety | - Comprehensive data on safety of biocide, including risk assessment data |
Term of validity for biocide approval
| Valid Term | Criteria |
|---|---|
| 10 years | - Biocides in general |
| 7 years | - Biocides which have had relaxed approval standards applied - Biocides to which respiratory organs are sensitively affected - Biocides which possess two or more out of persistence, bio-accumulativeness, and toxicity |
| 5 years | - Biocides which meet all three criteria qualifying for 7 years of validity |
Recognition of substance comparability
- Meaning| Chemical composition, risks and effects/efficacy such as removal of harmful organisms being technically comparable between different biocidal substances
- If comparability with a prior approved biocide(existing biocide) is proved and this fact is deliberated upon and recognized by the administration committee, the substance in question is considered to have been approved
- In such cases, consent must be obtained regarding use of data
Subjects excluded from requiring approval
| Group | Contents |
|---|---|
| Excluded from requiring biocide approval | - Biocides in general - Biocides which have been recognized as having low risk, and have been deliberated upon by committee and notified thus by the Minister of Environment - Biocides used in biocidal products utilized in experiments/analyses or research - Biocides used in biocidal products which are prototypes not intended for sale - Biocides decided by presidential decree, such as biocides which are entirely exported, etc. |