
Individuals wishing to manufacture or import biocidal products for domestic sale or distribution must receive approval for each company/product. Products containing existing biocides must receive approval within 2 years(2024~2031) from the expiry date of their respective biocide approval grace periods.
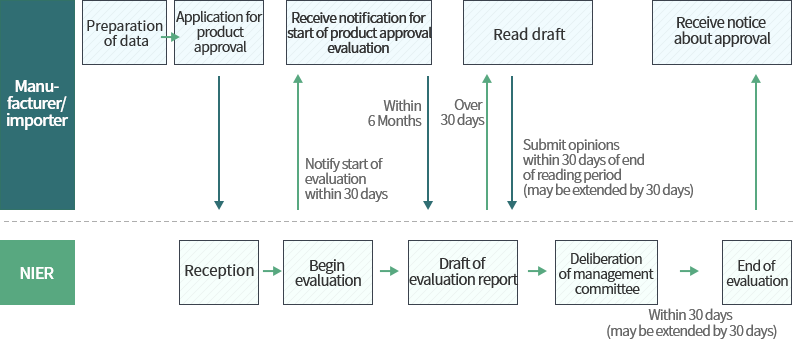
Process of biocidal product approval
Types of biocidal products
| Class | Type of Biocidal Product | Definition |
|---|---|---|
| 1. Disinfectants | Disinfectants | Disinfecting, sterilizing, pasteurizing, antimicrobial, etc. |
| ex) Disinfectants for objects, disinfectants for disease control | ||
| Algicides | Suppressing the growth of algae in water and eventually eliminating them (excluding products used in public waters) | |
| ex) Algae control chemicals in water parks | ||
| 2. Pest control products | Rodenticides | Removal of rodents such as rats |
| ex) Rat poison | ||
| Control products for other vertebrates | Removal of harmful vertebrates excluding rodents | |
| ex) Piscicides, avicides | ||
| Insecticides | Removal of insects such as flies, mosquitoes, ants, cockroaches, mites, etc. | |
| ex) Aerosolized insecticides, mothballs | ||
| Control products for other invertebrates | Removal of harmful invertebrates excluding insects | |
| ex) Molluscicides | ||
| Repellents | Neutralizing/suppressing harmful organisms by repelling them (excluding products used directly on the human body) | |
| ex) Anti-mite patches | ||
| 3. Preservatives | Product preservatives | Storing/preserving products to guarantee their expiry date |
| ex) Paint preservatives, food preservatives | ||
| Surface treatment preservatives | Coating product surfaces to preserve its initial state | |
| ex) Coating preservatives, anti-corrosives | ||
| Preservatives for fibers, leather, etc. | Preserving fibers, leather, rubber, etc. | |
| ex) Leather preservatives, air conditioner filter antibacterial agents | ||
| Wood preservatives | Preserving wood and products made of wood | |
| ex) Wood preservatives | ||
| Construction material preservatives | Preserving construction materials other than wood, such as stone or composite materials | |
| ex) Mold-proofing agents for construction materials | ||
| Material/Equipment preservatives | Preserving materials, equipment, structures, liquids, fluids, etc. used in industrial processes | |
| ex) Coolant preservatives, cutting fluid preservatives | ||
| Embalming/taxidermy preservatives | Preserving part or all of dead bodies of humans or animals | |
| ex) Taxidermy preservatives | ||
| 4. Miscellaneous | Antifoulants for ships/underwater equipment | Suppressing the fouling of ships, aquaculture equipment, underwater structures, etc. by harmful aquatic organisms |
| ex) Antifoulants |
Data required in biocidal product approval application
| Serial No. | Submitted Information | Details |
|---|---|---|
| 1 | Information of manufacturer/importer | - Name, trade name, address and contact information of applicant |
| 2 | Information of product | - Product name, type of biocidal product |
| 3 | Information of contents of product | - Composition, mixture ratio, purpose and uses for all substances in product - Name and address of supplier of biocide - If nanomaterials are included by design, name, purpose and uses of said nanomaterial(s) |
| 4 | Information on product | - Physical/chemical/biological properties - Exposure information(uses, main exposure channels, form of exposure, etc.) - Hazard/risk information regarding health/animals/ environment - Effects/efficacy - Classification, labeling and packaging |
| 5 | Data proving relaxed approval | - In case one or more relaxed approval conditions are applicable to product, data which verifies said fact |
| 6 | Raw materials and processes used in manufacture of biocidal product | - Raw materials and processes used in manufacture of biocidal product - Handling precautions, disposal methods for biocidal product - Information for domestic/overseas use and restrictions imposed on biocidal product - Data verifying use of safety container or packaging compliant to quality control obligation standards - Current status and future plans regarding compliance of manufacturing/storage facilities - In case of person(s) commissioning the manufacture of biocidal product, documents which verify said act such as copy of commission contract, etc. |
| 7 | Comprehensive data on safety | - Comprehensive data on safety of biocidal product, including risk assessment data |
Interim measures
Manufacturers/importers of biocidal products may manufacture/import them without receiving approval until their respective interim measure periods end.
| Group | Classification according to interim measures | Interim measure period |
|---|---|---|
| 1 | If all biocides in biocidal product are existing biocides subject to grace period for approval | Same as grace period for approval period for biocide (If there are two or more different existing biocides with differing periods, take the end date of the last postponement period) |
| 2 | If all biocides in biocidal product were subject to grace period for approval, but one or more substances were deemed unsuitable for approval and were prohibited from manufacture/import, or had their designation as approval-postponed existing biocides cancelled | Within 1 year from the manufacture/import prohibition date or the designation cancellation date, whichever comes first. |
| 3 | Biocidal products which do not fall under either Group 1 or 2 | Until December 31st, 2020 |
| 4 | Individuals manufacturing/importing biocidal products without approval as detailed in Groups 1~3, but who have received approval within the period given by each respective group | Until 1 year from the date the product was approved, the biocidal product may be manufactured/imported without being compliant to the labeled standards on its exterior |
Examples of interim measures
<Interim measures for manufacture/import of biocidal products>
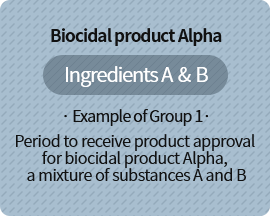
As the postponement period for ingredient B is longest at 5 years, 2 years are added to the substance approval postponement period of 5 years, and product must be approved within 7 years
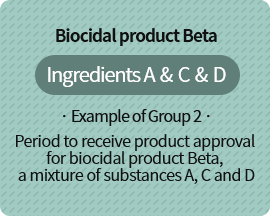
As substance C is the substance whose date of manufacture/import prohibition or designation cancellation comes first, product must be approved within 1 year from 2021.3.1.
As a biocidal product which does not correspond to Group 1 nor Group 2, product must be approved by 2020.12.31.
| Ingredients | Notice Contents |
|---|---|
| A | Existing biocide subject to approval postponement (approval postponement period: 3 years) |
| B | Existing biocide subject to approval postponement (approval postponement period: 5 years) |
| C | Substance prohibited from manufacture/import starting 2021.3.1. |
| D | Substance whose existing biocide designation is cancelled starting 2021.7.30. |
| E | Non-postponed substance (Biocide that was not notified as an existing biocide) |
Valid term for biocidal product approval
Individuals wishing to continue manufacturing/importing biocidal products after expiry of the product’s approval’s valid term must obtain approval again before said term ends.
| Valid term | Classification |
|---|---|
| 10 years | •Biocidal products in general |
| 5 years | • Biocidal products to which relaxed approval standards are applied • Biocidal products containing substances which are subject to relaxed substance approval standards |
| 3 years | •Biocidal products which meet both criteria qualifying for 5 years of validity |
Recognition of product similarity
- Meaning| The same biocides being contained between two different biocidal products, and substance composition/mixture ratio, uses, risks and effect/efficacy regarding harmful organism removal in the two products being similar to each other
- If similarity with an approved biocidal product(existing biocidal product) is proved and this fact is deliberated upon and recognized by the administration committee, the product in question is considered to have been approved
- In such cases, consent must be obtained regarding use of data
Special exceptions for product approval
| Conditions | |
|---|---|
| Applications, procedures and alterations for product approval excluded | 1. All component biocides are those notified as having low risk 2. None of the biocides are priority substances, nanomaterials, or hazardous/risky persistent organic pollutants 3. Must have sufficient effect/efficacy in removal of harmful organisms, etc. 4. Does not require personal protective equipment when handling or using |